CMC Potency Solutions
Your partner in CMC and functional assay excellence
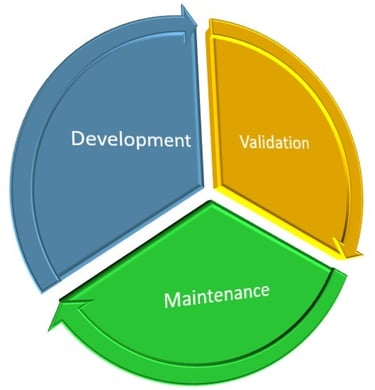
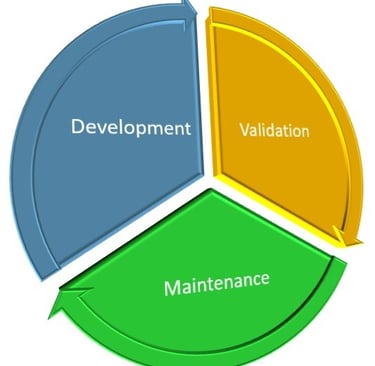
Potency Assay Life-Cycle Management
Strategic consulting on proof-of-concept, stage-specific design, development, qualification, validation, risk assessment, characterization, optimization, and troubleshooting. This includes:
Designing and tailoring programs for reference standards, control samples, and critical reagents, including analytical cell banks and their characterization/ qualification;
Leveraging DoE principles to improve efficiency in various stages (pre-clinical to commercial) and tasks (screening, robustness, validation, OOS investigation, etc.);
Designing and navigating method transfer and comparability studies, including transfers for academic/R&D labs and outsourcing contract labs;
Setting phase-appropriate specifications for in-process, release, and stability testing;
Preparing CMC sections (Module 3) of regulatory submissions, storyboards/briefing books, and addressing FDA inquiries;
Bioassay training for individuals and cross-functional teams, tailored to enhance technical proficiency and collaborative effectiveness.


Method Evaluation and Technical Support
Providing in-depth method evaluation with on-site support (optional) to minimize compliance risks and long-term complications. This includes:
Selecting the right platform and readouts (e.g., ELISA vs. SPR, cAMP vs. ion-flux);
Implementing appropriate controls (e.g., assay and quality control samples, specific enzymatic substrates, synthetic cells, etc.);
Utilizing representative models for data analysis and reporting results (e.g., slope ratio, parallel-line, 3-, 4- or 5-parameter logistic models);
Incorporating outlier detection strategies, including customization of SoftMax Pro templates for model-based algorithms;
Integrating data tracking and trending tools to enhance measurement process visibility, problem detection, and risk mitigation.


Extended Toolkit for Specialized Needs
In addition to core potency method development and lifecycle support, I share me expertise within a range of specialized services designed to strengthen overall product's quality and assay reliability, regulatory compliance, and operational efficiency:
Immunogenicity assays: support for developing and validating ELISA-based methods targeting host cell proteins (HCPs), residual DNA, residual Protein A, and total protein;
Documentation support: authoring, gap analysis, and evaluation of working instructions, method procedures, SOPs, protocols, and technical reports to ensure clarity and compliance;
Potency tailored audits: on-site and remote audits focused on the technical integrity and execution of potency assays, identifying risks and improvement opportunities;
Computerized system validation: preparation of User Requirements Specifications (URS) and Data Specifications (DS), along with support for Installation, Operational, and Performance Qualification (IOPQ) of computerized systems and analytical tools including Excel-based templates for streamlined potency data analysis and trending.
I’m a big fan of DeWALT for its consistency and reliability. (And no, this isn’t an ad)
Even with my fondness for Jack Sparrow’s antics, this is a line we can’t cross.
From a practical workload perspective, I’d estimate that development comprises over 50%, maintenance around 35%, and qualification/validation approximately 15%
FAQ
What services do you offer?
CMC solutions tailored to the specific client's need to establish a bullet-proof 'ed and stage-specific functional assay(s) for various biopharmaceutical therapies.
How do you ensure quality?
Beyond applying Quality-by-Design principles and a biology-first approach, I’m guided by a simple yet powerful question: “Would I treat my own children with the medicine released by [this] potency assay?” That mindset pushes me to think beyond the standard playbook and to ensure quality is deeply embedded in every practical and timely detail that matters.
What types of assays do you conduct?
Enzymatic assays, immunoassays, cell-based assays, and in vivo animal models to assess potency attributes across diverse modalities. What excites me most is the opportunity to support emerging therapies, especially site-specific or individualized treatments, while navigating the current regulatory framework and applying basic statistical principles (I am not a statistician, but I love to learn and dive into the data analysis to listen the voice of measurement process). Each new modality brings unique challenges, and helping shape its path to compliance and clinical impact is deeply rewarding.
Yes, biosimilars and related therapies including generics are integral part of my portfolio.
Can you assist with biosimilars?
Do you support gene therapies?
Absolutely, I’ve had the privilege of working on gene and cell therapies in the past and continue to do so through current projects, ensuring the development of compliant and robust functional assays. I’d be happy to contribute and learn alongside the team. Each novel product is a unique journey, often filled with unexpected challenges. That’s precisely what makes this field so compelling, it’s an opportunity to apply the foundational principles laid out by Professor David J. Finney in real-world, high-impact settings.
Where can I learn the basics if I’ve just started working on potency methods?
There are many excellent online resources and in-person learning opportunities available, including conferences such as BEBPA, workshops, and specialized courses. Personally, I highly recommend the FasTrain platform, which offers comprehensive coverage of CMC potency topics ranging from statistical approaches to GxP compliance. I also teach the CMC Relative Potency Analytical Methods: A Technical Deep Dive course there, designed to provide practical insights and advanced understanding for professionals working in this space.
You are very welcomed to explore the BEBPA and FasTrain portfolio here:
